In the US IDE clinical trial, monocular and binocular mesopic and photopic contrast sensitivity (with and without glare) was assessed at 6 Months.
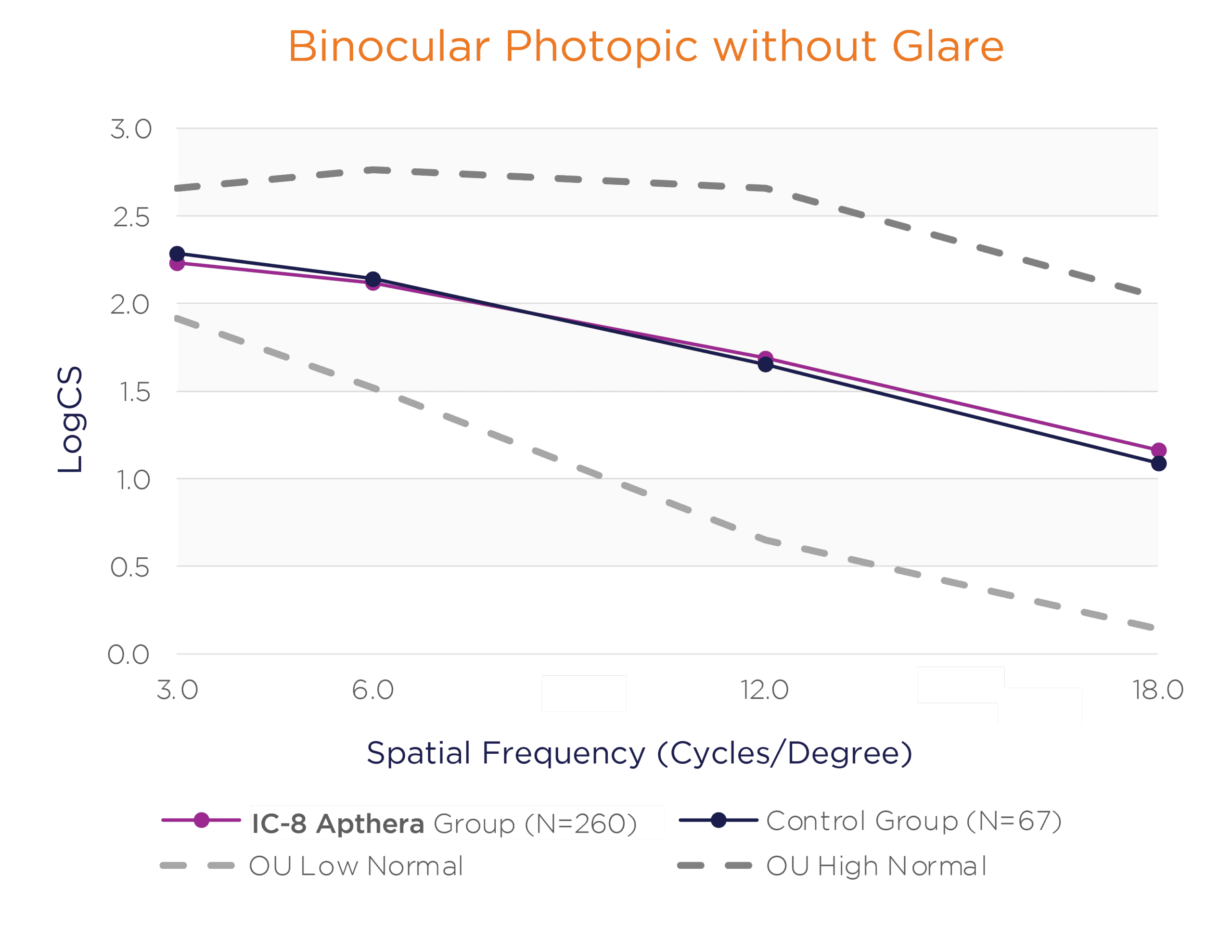
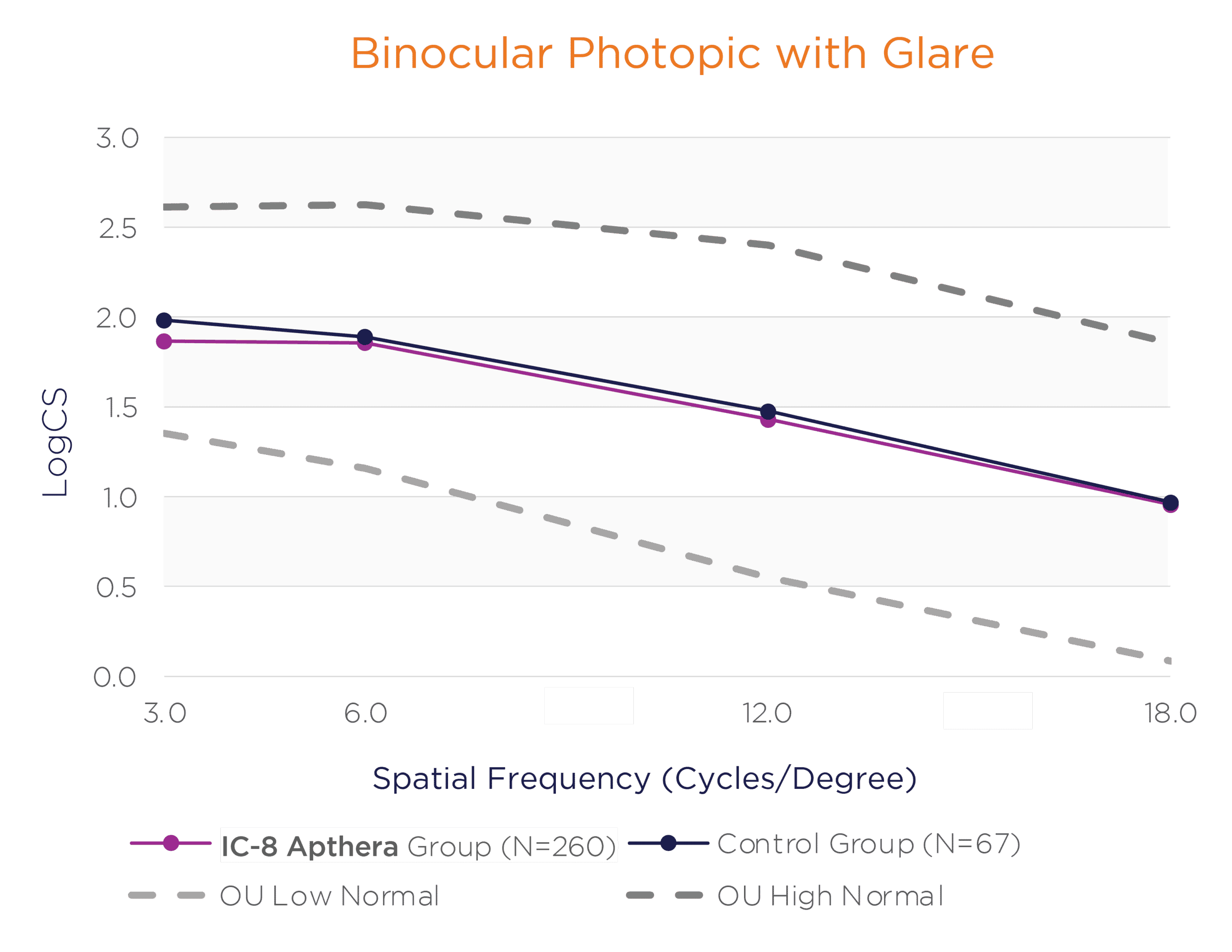
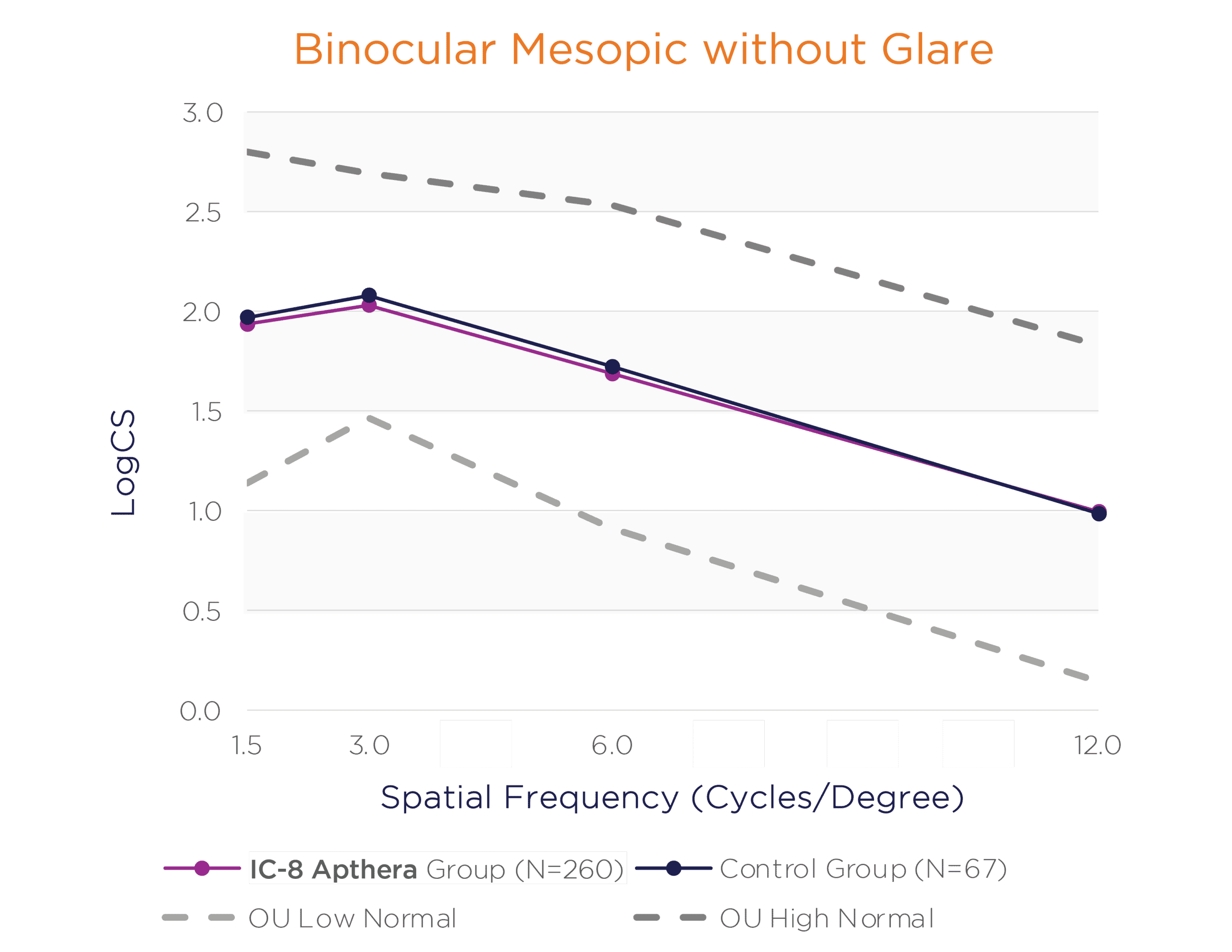
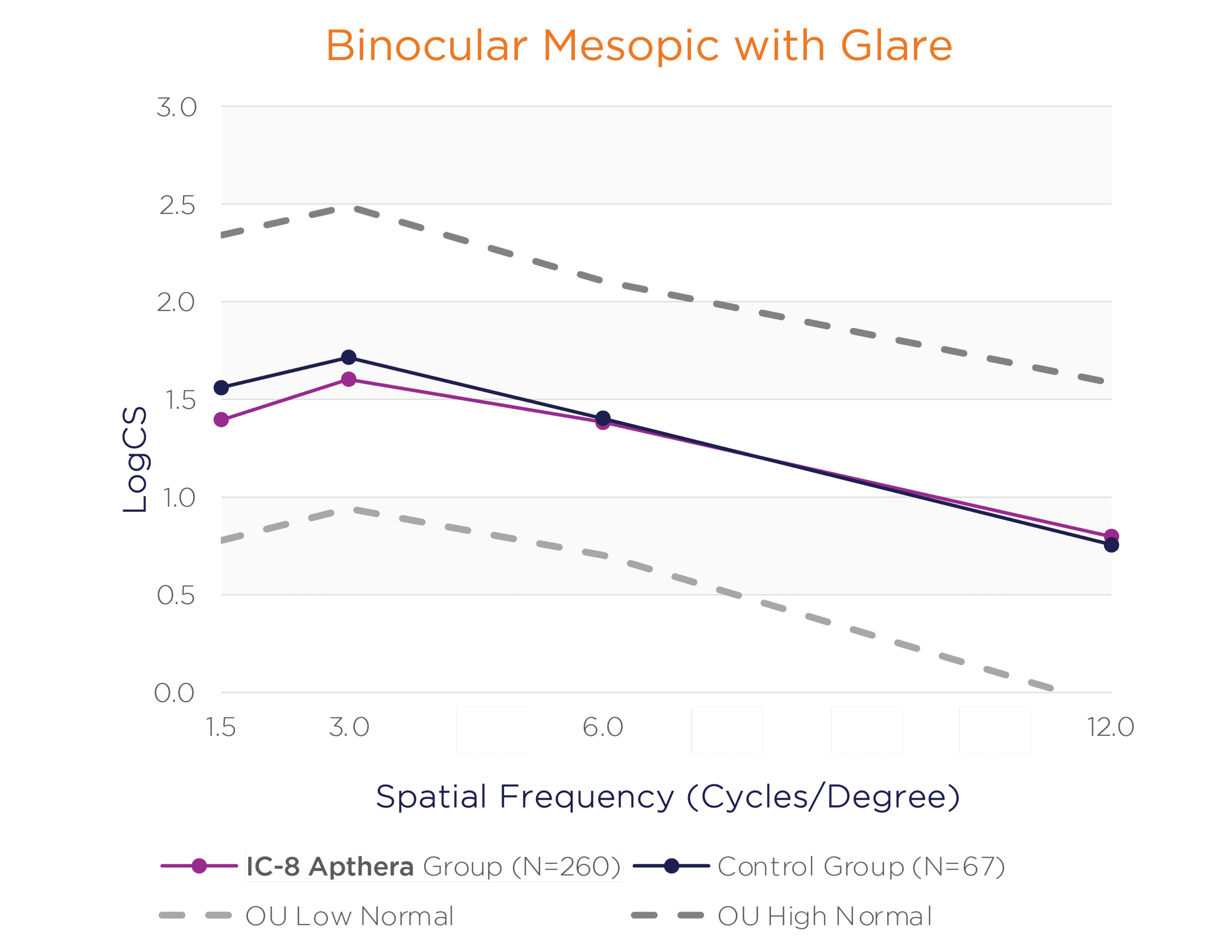
In the binocular natural viewing condition, in both mesopic and photopic conditions with and without a glare source, the IC-8 Apthera IOL subjects achieved similar mean contrast sensitivity compared to the monofocal/monofocal toric IOL subjects.
Binocular Photopic With and Without Glare Contrast Sensitivity (logCS) for IC-8 Group vs. Control Group at 6 Months
 |  |
Binocular Mesopic With and Without Glare Contrast Sensitivity (logCS) for IC-8 Group vs. Control Group at 6 Months
 |  |
A copy of the IC-8 Apthera IOL specifications sheet is available for download here.
The small aperture mechanism of action creates a defocus curve that includes up to 1.5 D of astigmatic refractive error. In the US IDE clinical trial, monocular uncorrected distance visual acuity was assessed in IC-8 Apthera IOL eyes with < 1 D of preoperative corneal astigmatism (Astigmatism Group 1) and compared with IC-8 Apthera IOL eyes with 1 D to 1.5 D of preoperative corneal astigmatism (Astigmatism Group 2). Astigmatism Group 2 was statistically non-inferior to Astigmatism Group 1 in monocular UCDVA (mean difference of 0.023 logMAR; p <0.0001), supporting that the IC-8 Apthera IOL provides consistent correction of aphakia with 20/25 average uncorrected distance vision for patients with up to 1.5 D of preoperative corneal astigmatism.
In the US IDE clinical trial, the Investigators rated 100% of the dilated fundus photography and dilated SD-OCT macular scan and disc scan image quality as excellent or adequate preoperatively and at 3 months, regardless of dilated pupil size. Additionally, at 12 Months, investigators reported little to no difficulty in 99.7% of IC-8 Apthera IOL eyes when performing retinal evaluation of optic disc and macula during dilated fundus examination using SLE.
